A new generation of purification technologies
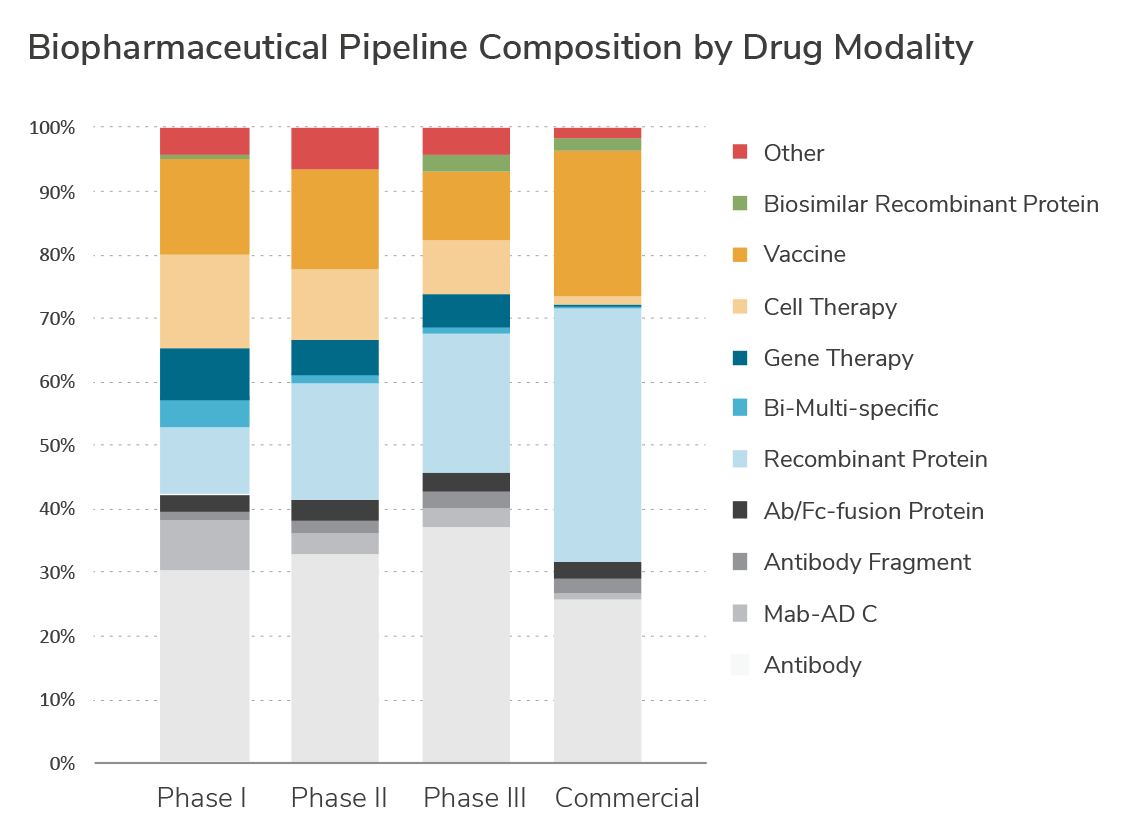
Biospecific Antibodies Fc Fusions
Growth Factors, Insulins, Hormones
Recombinant & Viral Vaccines
Gene Therapies
Cell-based Therapies
Product vs. Product-Impurity Separations
Process Analytical Technologies
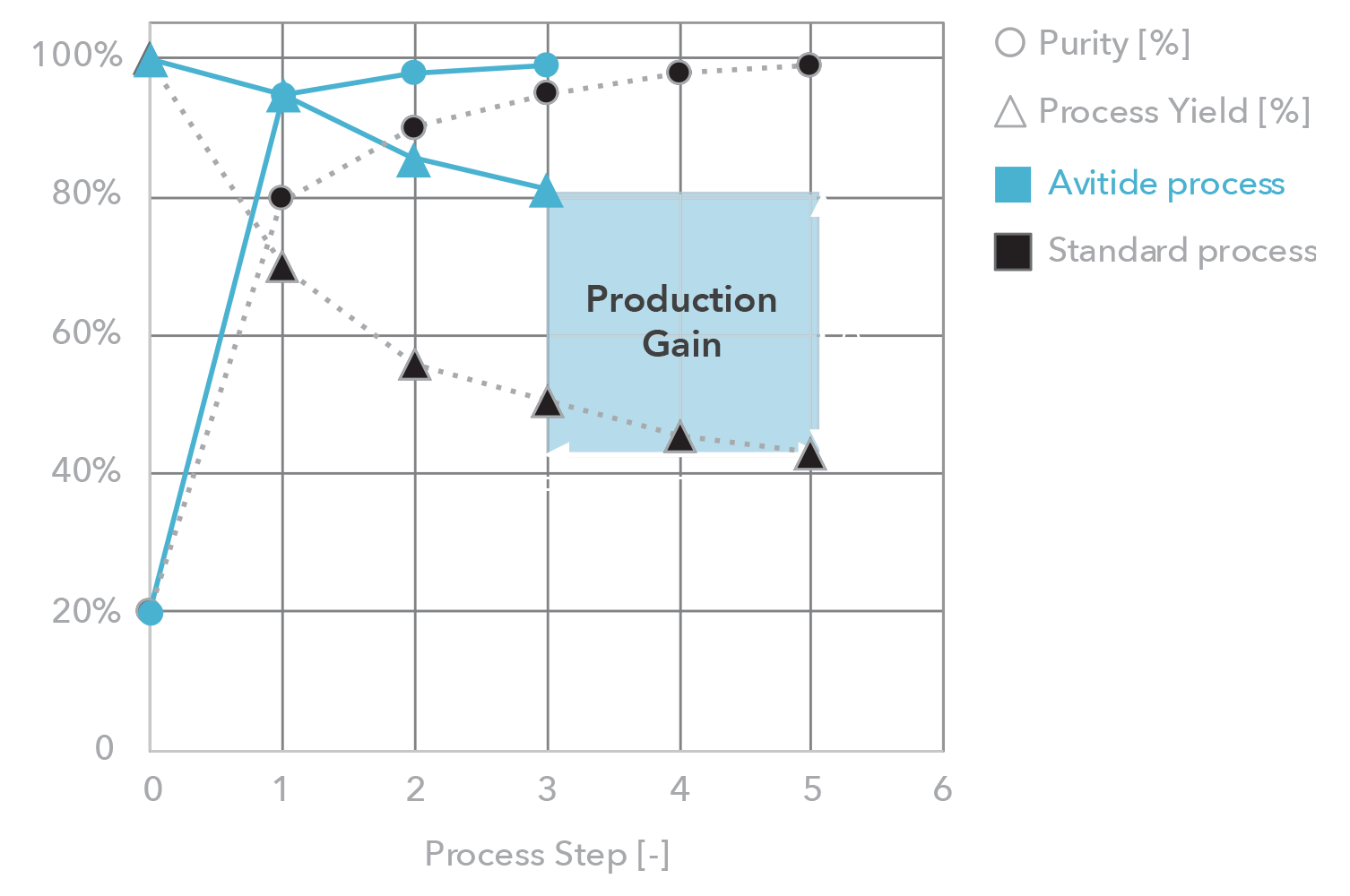
Affinity resins are affordable – Avitide partnerships have delivered
Providing reliable, consistent, and scalable manufacturing solutions
. . . . . . . . . . . . . . .
Ensuring purity and yield goals are met early in development
- Derisks commercial manufacturing line of sight
- Increases overall speed of development to reach the clinic and market
- Avoids development challenges commonly encountered with novel therapeutic modalities
- Integrates with CDMO development services
. . . . . . . . . . . . . . .
Improving commercial economic viability and drug accessibility through cost efficiencies
. . . . . . . . . . . . . . .
Expanding exclusive intellectual property around biologics via new manufacturing process patents
. . . . . . . . . . . . . . .
Establishing multi-product facilities with similar operational architecture to antibody production facilities
. . . . . . . . . . . . . . .
Improving overall facility productivity and leverage of existing CAPEX investments
. . . . . . . . . . . . . . .
Enabling fully continuous downstream processing of novel biologic modalities
1-2 yrs
$4-6M
2-8x
2-6x
16 Cavendish Court
Lebanon, NH 03766
603.965.2100
email us
Subscribe to hear the latest news and insights…
